I hope you
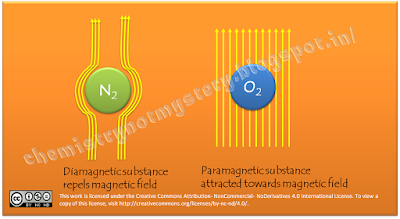
have seen the video of the link I had given you in previous post. Did you
notice that Oxygen somehow dances between the poles while Nitrogen escapes?
This strange behaviour can be explained by MOT.
You can see
Oxygen gets attracted toward the magnetic field while Nitrogen repels it. In
both cases N2 and O2 behave like a magnet. You
may get surprised here and ask, how can I relate a molecule to a magnet? You
must have learnt in physics that magnetic field is associated with moving
charged particle. Similarly in molecules moving negatively charged electrons
generate a magnetic field. Thus, the magnetic behaviour of an atom or a
molecule is related to the orbital and spin motion of its electrons. Quantum
number ml and ms represent the
magnetic factor of an electron.
That means
the reason behind their strange behaviour lies in their electronic arrangement.
So, have a look on electronic configuration of N2 and O2
again.
Electronic
configuration of N2: σ1s2, σ*1s2, σ2s2,
σ*2s2, {π2py2, π2pz2}, σ2px2
Electronic
configuration of O2: σ1s2, σ*1s2, σ2s2,
σ*2s2, σ2px2, {π2py2, π2pz2},
{π*2py1, π*2pz1}
You can see
that in N2 all electrons are paired while O2 has 2
unpaired electrons. These unpaired electrons are responsible for the magnetic
nature of O2.
These
unpaired electrons of O2 move around in their orbits. Their
orbital motion generate loop of current which produces magnetic field. You may
think that both unpaired electrons spin in clockwise direction so their
magnetic field will add to give a strong resultant magnetic field which makes O2 a
powerful magnet. But it doesn't happen because these electrons are randomly
arranged in a molecule so they cancel each-other's magnetism and very little
magnetism is left within a molecule.
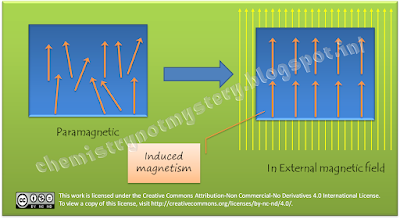
As we apply
external magnetic field these tiny magnets get aligned in the same direction as
the external magnetic field thus they produce induced magnetism in the
direction of applied field which is proportional to the applied field. That's
why O2 get attracted towards external magnetic field. This type of
magnetism is called paramagnetism.
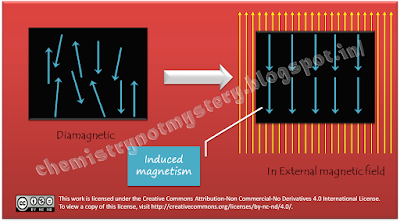
In case of N2
molecule, all electrons are paired. That means half of the electrons spin
clockwise and half of the electrons spin anticlockwise. Because of their opposite
spins they produce magnetic field in opposite direction thus the resultant
magnetism becomes zero. When such molecules are placed in an external magnetic
field they produce induced magnetic field in opposite direction and that's why
they repel the magnetic field. This type of magnetism is called diamagnetism.
Actually all
atoms/molecules are diamagnetic inherently but the presence of unpaired
electrons produces some magnetism in the atoms/molecules and make them
paramagnetic. Paramagnetic molecules get attracted towards external magnetic
field and diamagnetic repel the external magnetic field.
You can
easily predict the magnetic nature of any molecule/atom by its electronic
configuration. If it has any unpaired electrons it will be paramagnetic and
otherwise it will be diamagnetic. In the next
post we will see how MOT deals with the molecules formed by two different
elements.
Related Posts-
Click to subscribe YouTube channel chemistrynotmystery
https://www.youtube.com/channel/UCDuIUfNXOtkXS1V-nl2L
This work is licensed under the Creative Commons
Attribution- NonCommercial- NoDerivatives 4.0 International License. To view a
copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.

View comments